A new study published on 21 September in Joule has demonstrated a new approach that could potentially combine carbon dioxide (CO2) capture and conversion in a single device (1). The researchers from the Massachusetts Institute of Technology (MIT) have developed a novel chemistry for post-combustion CO2 capture and conversion. The electrochemical CO2 conversion is achieved using only a carbon electrode, thereby effectively combining two conventionally separate approaches to CO2 mitigation.
Carbon capture involves collecting waste CO2 emissions from large point sources, such as fossil fuel power plants, and preventing them from entering the atmosphere. This typically involves converting CO2 into specialized chemicals via metal catalysts, which is an expensive energy-consuming process. At present, carbon capture technologies can capture more than 90 per cent of CO2 emissions from power plants and industrial facilities, however, the process generally uses up to 30 per cent of the electricity generated by the plant. Therefore, huge efforts are being made toward the development of post-combustion CO2 as a source of fuel based on chemical or electrochemical processes.
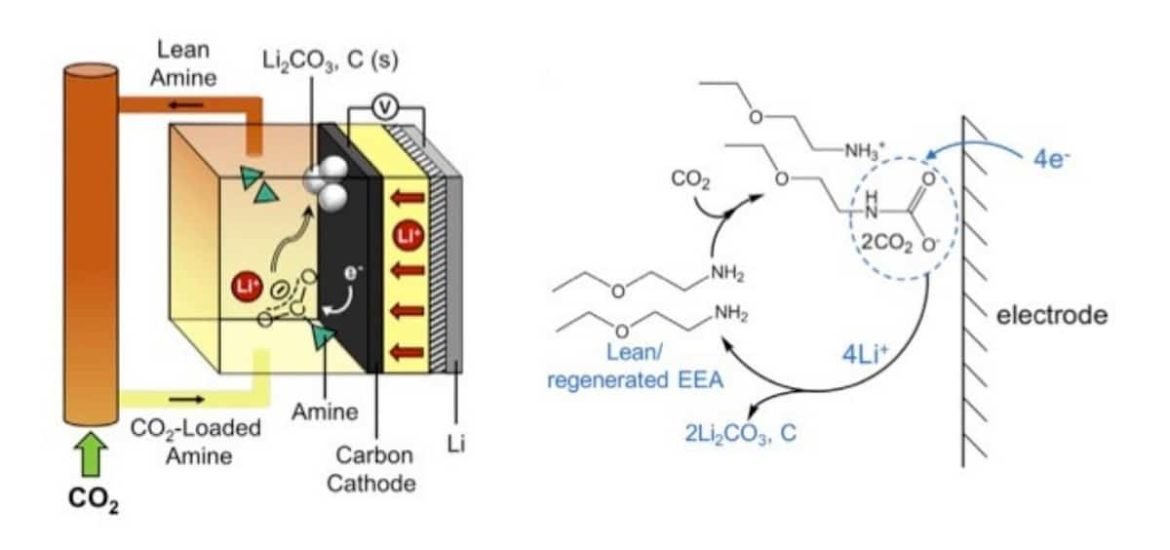
One promising idea is to produce lithium-CO2 batteries that use CO2 as a reactant during discharge, however, efforts to make use of captured CO2 in energy storage devices such as batteries have been hindered by the low electrochemical activity of CO2. The process typically requires the use of expensive metal catalysts and the reactions are difficult to control. According to the authors, CO2-capture chemistry could be put to use to make CO2-loaded electrolytes, an essential part of a battery. The captured gas would be used during the battery discharge process to provide a power output.
The battery is made of three main components: lithium metal, carbon, and an electrolyte. The researchers write that by “adding a CO2 capture agent such as an alkyl amine into an organic, Li+-containing electrolyte yields a new redox-active species that can be directly reduced at a catalyst-free carbon electrode in a Li-CO2 battery with high discharge voltage and capacity.” In other words, by incorporating the gas in a liquid state, Gallant and her team have found a way to achieve electrochemical CO2 conversion using only a carbon electrode. To achieve this, the CO2 is ‘‘pre-activated’’ by incorporation into an amine solution, thus avoiding the classical electrochemical CO2 reduction.
The proof-of-concept has shown that the approach does works and can produce a lithium-carbon dioxide battery with voltage and capacity that are comparable to state-of-the-art lithium-gas batteries. Further optimisation is still needed as the battery is currently limited to 10 charge-discharge cycles and to prevent degradation of the battery components.
Although the research is still in the early stages and a lithium-CO2 battery is probably years away, the concept could potentially lead to new battery formulations with tailored electrochemical CO2 conversion reactions to help mitigate greenhouse gas emissions to the atmosphere. The ultimate aim would be to develop an integrated system that can both capture CO2 from a power plant and convert it into an electrochemical material that could be used in batteries.
(1) Khurram, A., He, M., and Gallant, B.M. Tailoring the Discharge Reaction in Li-CO2 Batteries through Incorporation of CO2 Capture Chemistry. Joule (2018) DOI: 10.1016/j.joule.2018.09.002
Image: Khurram et al. Joule (2018)