Despite the binary thinking of progressive scientists on the one hand and prophets of doom on the other, science doesn’t tend towards good or evil. Any experiments scientists can do, they will. This is a positive thing, because who is a position to claim omniscience and to forbid it? In fact, it’s human beings who choose to use the results of science and in so doing retain some of them and eliminate others. This responsibility is both personal and collective[1] . It is not so much about discovery as intervention, mainly in medicine, but also in dissemblance or fraud.
Negative clinical trials represent valuable information
That is why it is useful, and also honest and responsible, to report negative results of clinical studies. This move towards full transparency in clinical studies in medicine in order to be able to perceive a full and true picture of the results of a drug or treatment technology has been fostered by the prior declaration of clinical trials worldwide. It was not triggered by law, but by an incentive linked to publication criteria. So, a clinical trial that has not been reported on before it begins is unlikely to find its way into a high-level publication. In some countries the ethics committee will only make a ruling when a declaration to this effect is included in the draft. For those interested in clinical trials, the main site listing these trials is: https://clinicaltrials.gov/. This database allows us to explore 301,373 trials in 209 countries to date. Clinicaltrials.gov is a resource provided by the National Library of Medicine in the United States. Knowing about a negative clinical trial provides a range of information to the scientific community. First of all, the possibility of reconsidering the indications for treatment if patients are already being treated with the method tested. Then, directing the research and allocation of funds to other avenues, or other goals.
Stem cells in heart failure
Stem cells have attracted the attention of scientists for a long time and the ability to manipulate them has aroused great hopes in the cardiovascular field[2]. In particular, in the area of heart failure, whether it is congestive heart failure or the aftermath of myocardial infarction, there has been widespread take-up of the hypothesis that dedifferentiated cells can regenerate the heart and avoid the need for circulatory assistance devices or transplants. The first “evidence” of cardiac stem cells in adult organisms appeared in the early 2000s, when biologists announced that cells derived from the bone marrow or adult heart expressing the c-kit protein could create new muscle tissue when injected into a damaged myocardium. These studies were conducted in rodents. They faced challenges from the very start because we already know that the human heart does not repair itself after a heart attack. In this context an important clinical trial has just been published by the JAMA (10.1001/jama.2019.2341).
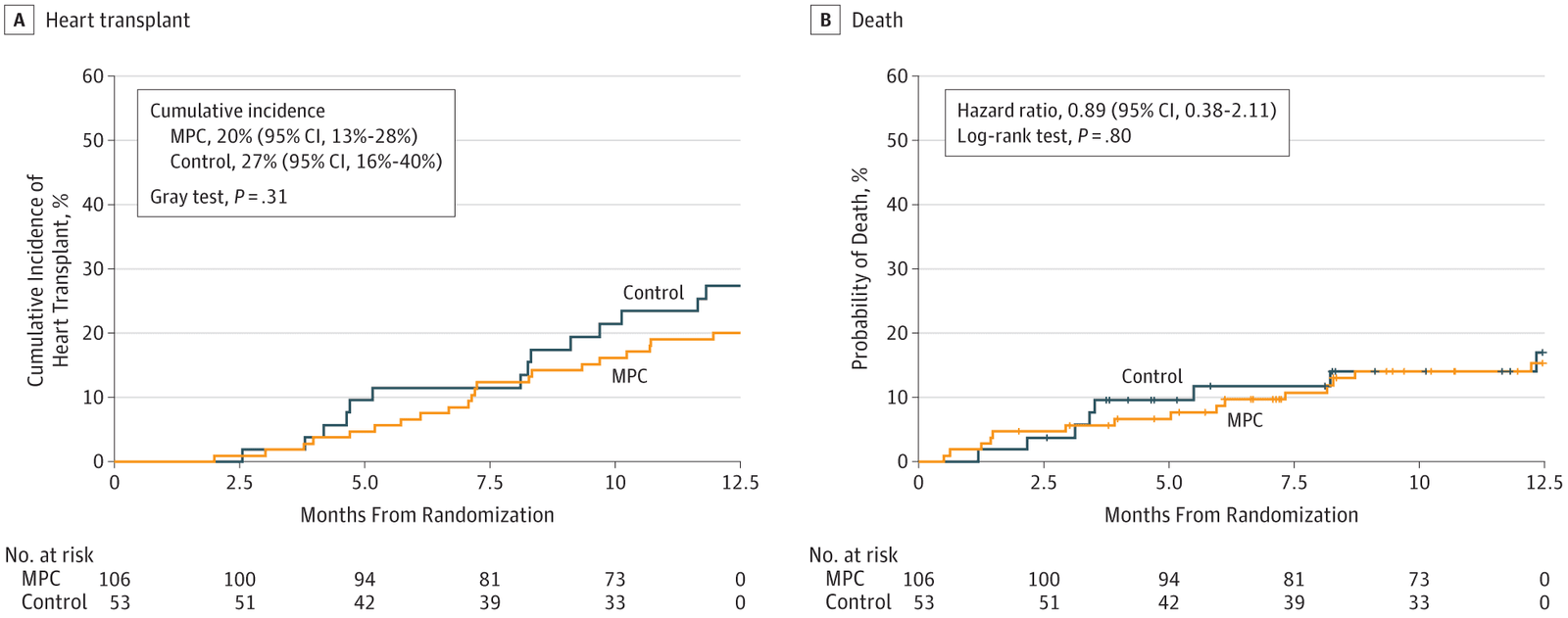
Figure 1. On the left the probability of heart transplant in the control group control in the stem cell group, on the right the deaths in both groups over a one-year period. While the curve on the right clearly demonstrates the number of deaths after 1 year with a simple equivalence, the one on the left requires statistical interpretation. Is the difference observed simply random or is it as a result of treatment? In this case the statistical test allows us to conclude with only a 5% chance of misrepresentation that this is a random difference. In other words, given the percentage of cardiac transplants in both groups one cannot reject the null hypothesis, which is the initial hypothesis i.e. that the treatment is ineffective (10.1001/jama.2019.2341 ).
The results of this test are very clearly illustrated in Figure 1. Stem cells injected into the heart do not improve survival and do not reduce the need to resort to transplantation. As the editor’s note in JAMA states factually:
“However, the weight of the evidence to date indicates that any clinical benefit resulting from stem cell therapy for heart failure with reduced ejection fraction has been small and inconsistent. The study by Yau et al5 in this issue of JAMA is yet another example of a negative study in a largely disappointing body of cell-based research. The investigators injected bone marrow–derived mesenchymal precursor cells into the myocardium of patients who were receiving LVAD support for advanced heart failure—106 patients received mesenchymal stem cells and 53 received sham injections—and found no benefit on the ability to wean the patients from LVAD support at 6 months. This study is important because it provides further evidence that mesenchymal precursor cells injected into the myocardium may not be the answer to the challenging problem of advanced heart failure due to dilated cardiomyopathy.”
It seems that the somewhat simplistic idea of injecting cells into an organ and hoping that these cells attach to the right place, differentiate and become functional is actually an illusion. But it does not seem to be just a disappointment, a concept that ultimately fails to fulfil its clinical promise. The results of the randomized controlled trial published in the JAMA may signal the end of the “heart stem cells” adventure, but it also allows us to begin to unravel some of the threads of a story fraught with fraud.
Heart stem cells feature at the centre of multiple cases of fraud.
To market their rejuvenating treatments, the great cosmetics brands bandy about terms like new cells, regenerating cells, anti-aging cells etc… The marketing spin is totally disconnected from reality but it riffs off a timeless idea. And several researchers have been caught in flagrante delicto committing fraud. “For the article entitled ‘Human cardiac stem cell differentiation is regulated by a mircrine mechanism.’ (Circulation. 2011; 123: 1287–1296; DOI: 10.1161 / CIRCULATIONAHA. 110.982918) an investigation by Harvard Medical School and Brigham and Women’s Hospital (BWH) has determined that there were issues with some of the data reported in the article, specifically Figure 7B. An expression of concern was published on November 20, 2018, to alert readers that the American Heart Association was communicating with the authors and reviewing the materials provided by BWH. After considering input from authors, the American Heart Association has determined that the best interest of the public and the research community will be served by issuing this notice of retraction. The American Heart Association, therefore, retracts the article.”
We admire the very restrained form of words but in reality, it relates to 31 articles from P. Anversa’s cardiac stem cell laboratory[3] which will be removed on the grounds of falsification or fabrication of data. The laboratory closed down in 2015 and the hospital will have to pay out $10 million to conclude litigation with the Department of Justice since the laboratory had obtained public funds. P. Anversa’s work is based on the idea that the heart contains stem cells that could regenerate the heart muscle[4] . He and his colleagues said that they had identified such cells, the c-kit cells. Various research teams attempted to replicate P. Anversa’s results but failed. Nevertheless, some scientists have tried to inject cells expressing c-kit protein into damaged hearts, with inconclusive results. “There are no stem cells in the heart. Stop trying to publish results on this subject.” J. Molkentin, who has questioned this concept from the beginning, is insistent. What is possible, however, is that some cells may be useful for repairing heart cells, but we do not currently have an efficient method of programming them; or that transforming a mesenchymal cell into a muscle cell is only possible in the embryo[5].
In 1746 Dr. J. Lind carried out one of the first randomized therapeutic trials that showed that citrus fruits cured scurvy[6]
Subsequently this type of trial has saved millions of human lives, for example by avoiding bleeding, despite this already being officially known, but also by highlighting the danger of certain medicines or surgical procedures… Many treatments or assertions in medicine stem from limited observations, received wisdom or knowledge confined to certain schools of thought. They date back to before the era of randomized controlled clinical trials and electronic dissemination of information. It is therefore very likely that there are still many mistakes, unfounded beliefs, or statements contrary to reality in medical practice. At the same time the rapid transformation of therapeutic tools can quickly render entire ranges of interventional drugs and treatment obsolete. As a result, it’s likely that we will never know that these treatments were absolutely ineffective because they have been superseded and forgotten, and no one will invest time and money to find out about them. On the other hand, it is extremely useful for patients, that current treatments without a solid basis should be identified and the results made known to everyone. It is about efficiency and value for money. And sometimes about discovering fraud.
Medicine based on the results of controlled and randomized trials is often set up in opposition to personalized medicine that supposedly does not need it. It’s exactly the opposite. The weakness of some medical recommendations is not related to clinical trials, nor is it related to evidence-based medicine, it is related to what might be called the underestimation of our differences. In future it is likely that genomics will allow clinical trials to be conducted not on an entire population, which often leads to a lowest common denominator use of information, but on populations with particular genetic characteristics. This is already the case with a number of monogenic genetic markers that tend towards Alzheimer’s disease, diabetes or cancer. The recommendations will then be tailored based on differentiation criteria that weigh heavily in terms of risk. Fortunately for some patients with severe heart failure there are now alternatives to “stem cells”, which may well be more onerous but nonetheless effective[7].
[1] https://www.atlantico.fr/decryptage/3567689/therapies-geniques–petits-reperes-pour-distinguer-celles-qui-pourront-nous-guerir-de-celles-qui-affecteront-la-nature-meme-de-l-homme-guy-andre-pelouze
[2] https://www.economist.com/science-and-technology/2011/11/19/repairing-broken-hearts
[3] https://retractionwatch.com/2018/12/13/anversa-cardiac-stem-cell-lab-earns-13-retractions/
https://www.statnews.com/2018/10/14/harvard-brigham-retractions-stem-cell/
[4] https://www.futuremedicine.com/doi/full/10.2217/17460751.1.2.153?select23=Choose&
[5] https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.118.034250
[6] https://www.pseudo-sciences.org/spip.php?article1651
[7] https://www.ahajournals.org/doi/10.1161/JAHA.117.006408
This post is also available in: FR (FR)