A paper published on 3 September in Nature Nanotechnology presents a new approach that could potentially be used to slow down the progression of rheumatoid arthritis (1). The neutrophil nanosponges, developed by Prof Liangfang Zhang and his team at the UC San Diego Jacobs School of Engineering, were shown to be safely absorbed in mice and neutralize a variety of proteins that play a role in the progression of rheumatoid arthritis. The common and often devastating chronic inflammatory autoimmune disorder causes painful swollen joints.
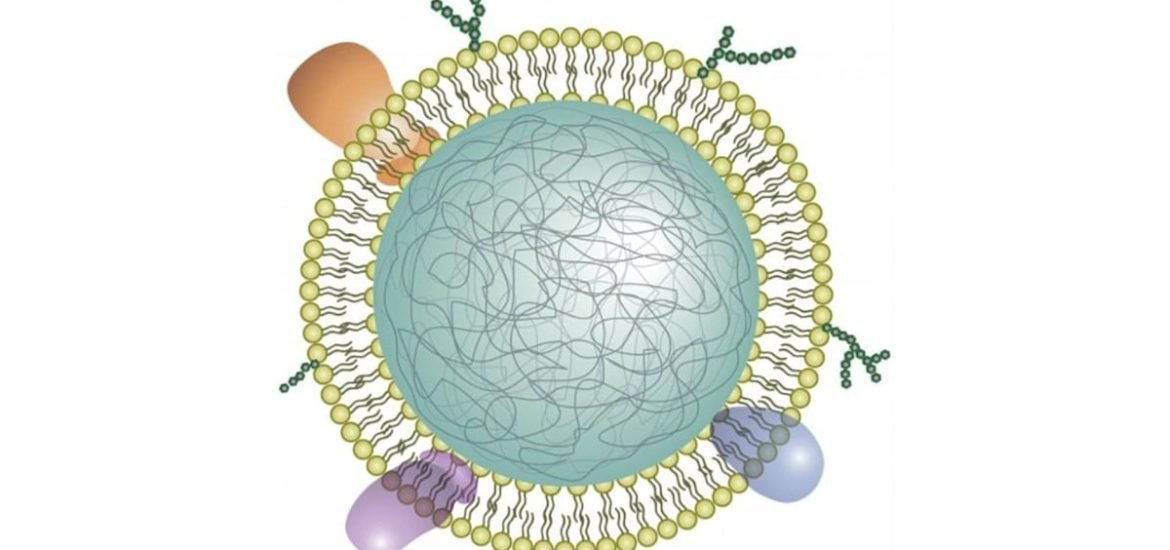
The nano-sized sponges are made from a biodegradable polymer coated with the cell membranes of neutrophils, a type of white blood cell. Neutrophils play an important role in rheumatoid arthritis, often referred to as “first responders,” the cells normally protect the body from invading pathogens by triggering inflammation. In the case of rheumatoid arthritis, however, for reasons that remain elusive, the inflammatory cascade is triggered in joint tissues due to the release a complex array of pro-inflammatory cytokines, ultimately leading to damage of cartilage and joint tissues.
To create the neutrophil nanosponges, the team first separated neutrophils from whole blood and placed them a solution that causes them to swell and burst, leaving behind only the cell membrane, which contains important receptor-binding proteins. The membranes were then broken up and fused with the polymer-based nanoparticles. Since the cell membrane contains the important proteins required to interact with cell receptors in the joint tissue, the resulting neutrophil nanosponges were expected to mimic real cells in certain functions.
To test their theory, the nanosponges were injected into the inflamed joints of mice in which severe rheumatoid arthritis had been induced and resulted in reduced swelling and also prevented cartilage damage. In addition to performing equally well compared to a high-dose of antibodies, the nanosponges also worked as a preventive when administered prior to inducing the disease.
According to the authors, the nanosponges were able to “neutralize proinflammatory cytokines, suppress synovial inflammation, target deep into the cartilage matrix, and provide strong chondroprotection against joint damage.” The nanosponges essentially suppress the inflammatory process by binding to neutrophil receptors (proteins) in tissue and preventing the cells from releasing more neutrophil-attracting cytokines.
Current treatments are based on antibodies that neutralise just one or two proinflammatory cytokines but due to a vast array of cytokines and complex signalling pathways involved in rheumatoid arthritis, these types of treatment are not hugely effective, particularly in severe cases. Neutrophil membranes, on the other hand, are capable of binding to receptors of all the different types of cytokines, and therefore, may be more effective at managing the entire family of cytokines.
“Nanosponges are a new paradigm of treatment to block pathological molecules from triggering disease in the body,” says Zhang, Ph.D., a nanoengineering professor at the UC San Diego Jacobs School of Engineering. “Rather than creating treatments to block a few specific types of pathological molecules, we are developing a platform that can block a broad spectrum of them, and this way we can treat and prevent disease more effectively and efficiently.”
(1) Zhang, Q. et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nature Nanotechnology (2018). DOI: 10.1038/s41565-018-0254-4
Image source: Zhang, Q. et al.Nature Nanotechnology, 2018.